-
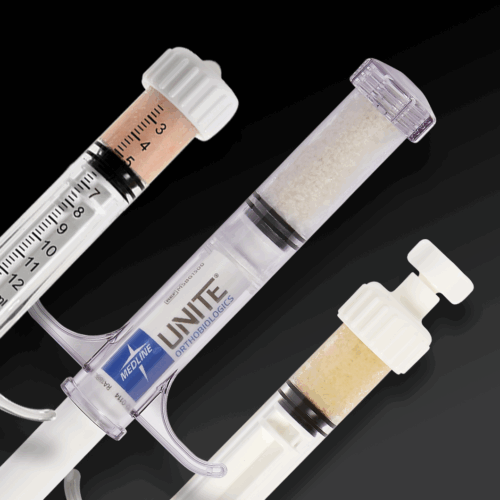
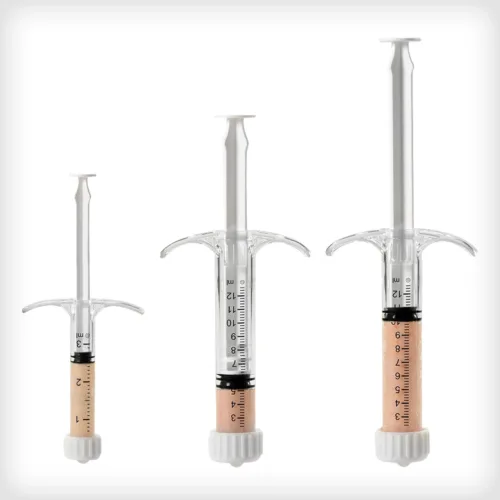
 ACTIGLASS™ Backfill Bioimplant combines the osteoconductive properties of tricalcium phosphate and hydroxyapatite with bioactive glass in a collagen matrix. Together, this biocompatible composition can resorb and be replaced by natural bone. This synthetic bioimplant is pre-sized for compatibility with the Autograft Harvester, allowing for improved intraoperative convenience and efficiency.
ACTIGLASS™ Backfill Bioimplant combines the osteoconductive properties of tricalcium phosphate and hydroxyapatite with bioactive glass in a collagen matrix. Together, this biocompatible composition can resorb and be replaced by natural bone. This synthetic bioimplant is pre-sized for compatibility with the Autograft Harvester, allowing for improved intraoperative convenience and efficiency. -
 Next-generation bone allograft solution with an average of 1.5 million viable cells per cc of allograft. Osteogenic, osteoconductive and osteoinductive properties provide the three key elements for bone formation. Proprietary, DMSO-free cryopreservation technology preserves an average of over 92% cell viability post-thaw and eliminates rinsing or decanting steps. Optimized handling characteristics and syringe packaging to allow for easy moldability and improved intraoperative efficiency.
Next-generation bone allograft solution with an average of 1.5 million viable cells per cc of allograft. Osteogenic, osteoconductive and osteoinductive properties provide the three key elements for bone formation. Proprietary, DMSO-free cryopreservation technology preserves an average of over 92% cell viability post-thaw and eliminates rinsing or decanting steps. Optimized handling characteristics and syringe packaging to allow for easy moldability and improved intraoperative efficiency. -

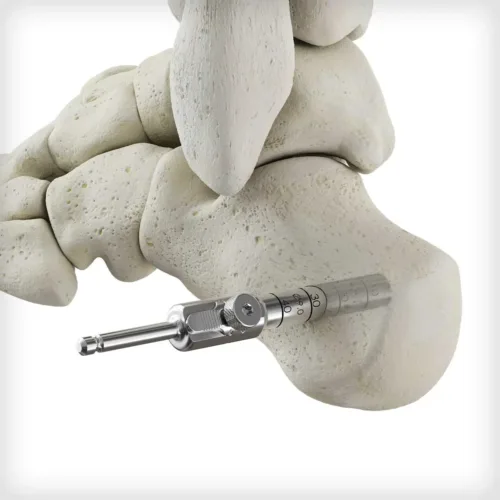
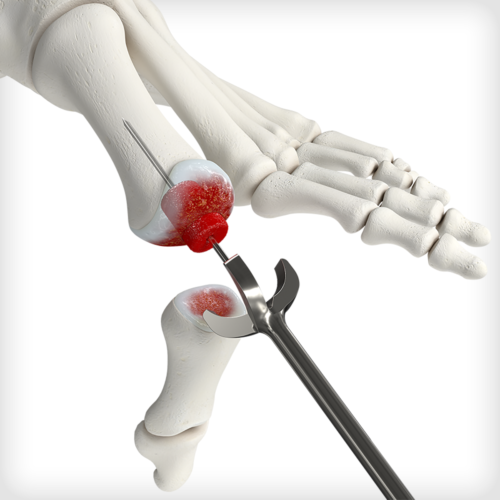 Dense cancellous MTP Revision Bioimplants are pre-hydrated in saline to eliminate idle OR time associated with cutting and soaking a traditional bone graft. The bioimplants are sterilized in saline rather than freeze-dried, to preserved the structural integrity of the graft and to avoid intraoperative graft fracture when reaming. Two distinct size options are available for unique revision scenarios: a small option designed to backfill metatarsal head voids and a large option design to bridge a larger gap and restore length. The bioimplants feature a flat-cut, cannulated designed to be used with a provided 1.6mm guidewire, MTP cup and cone reamers, and acorn reamer. Reamers are used for intraoperative shaping and graft site preparation, which may vary depending on revision scenario and surgeon-preference or technique.
Dense cancellous MTP Revision Bioimplants are pre-hydrated in saline to eliminate idle OR time associated with cutting and soaking a traditional bone graft. The bioimplants are sterilized in saline rather than freeze-dried, to preserved the structural integrity of the graft and to avoid intraoperative graft fracture when reaming. Two distinct size options are available for unique revision scenarios: a small option designed to backfill metatarsal head voids and a large option design to bridge a larger gap and restore length. The bioimplants feature a flat-cut, cannulated designed to be used with a provided 1.6mm guidewire, MTP cup and cone reamers, and acorn reamer. Reamers are used for intraoperative shaping and graft site preparation, which may vary depending on revision scenario and surgeon-preference or technique. -

 Dense cancellous Evans and Cotton Wedges are pre-shaped and pre-hydrated to eliminate idle OR time associated with cutting and soaking a traditional bone wedge. The wedges are sterilized in a solution rather than freeze-dried, to preserve the structural integrity of the graft and avoid intra- and post-operative crumbling. The system comes equipped with trials to determine the proper wedge size.
Dense cancellous Evans and Cotton Wedges are pre-shaped and pre-hydrated to eliminate idle OR time associated with cutting and soaking a traditional bone wedge. The wedges are sterilized in a solution rather than freeze-dried, to preserve the structural integrity of the graft and avoid intra- and post-operative crumbling. The system comes equipped with trials to determine the proper wedge size. -

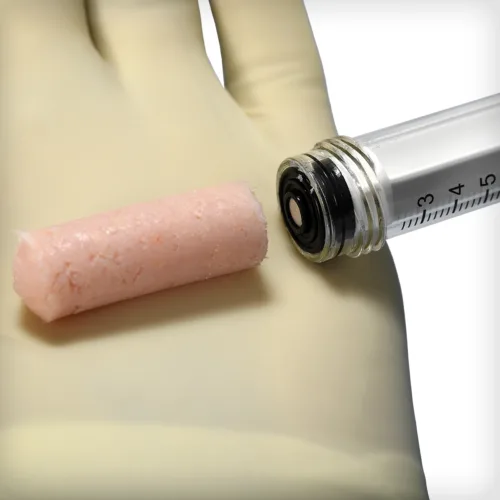
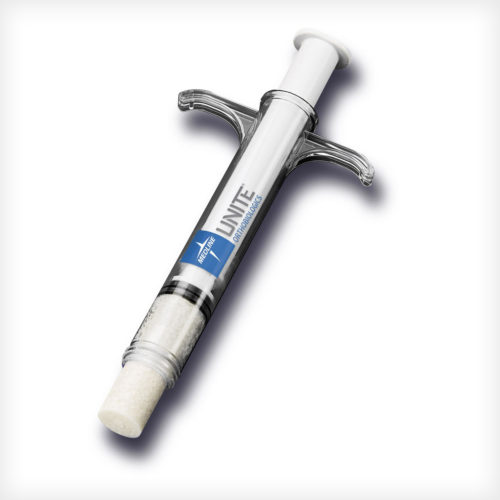 ACTIGLASS™ Synthetic Bioactive Putty combines the osteostimulative properties of bioactive glass with a dynamic biphasic mineral composite of beta tricalcium phosphate and hydroxyapatite granules. Together, these proven biomaterials respond to the body’s own regenerative process to support bone healing at all stages. The putty features a unique waxy texture that can be shaped and sculpted to fit any defect.
ACTIGLASS™ Synthetic Bioactive Putty combines the osteostimulative properties of bioactive glass with a dynamic biphasic mineral composite of beta tricalcium phosphate and hydroxyapatite granules. Together, these proven biomaterials respond to the body’s own regenerative process to support bone healing at all stages. The putty features a unique waxy texture that can be shaped and sculpted to fit any defect. -

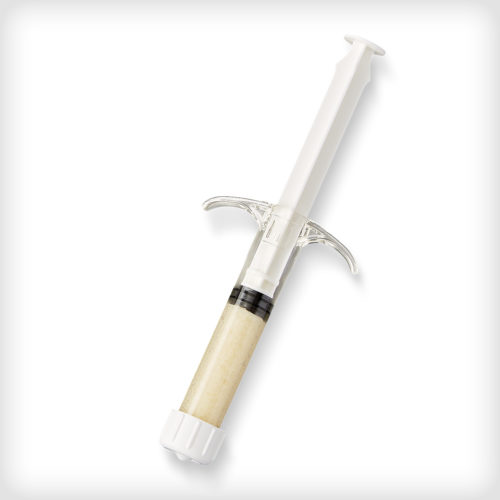 ACTISTIM™ Demineralized Fiber Putty is made from 100% human allograft. The putty features an osteoinductive and osteoconductive 3D interwoven demineralized cortical fiber scaffold for improved handling, wicking, and mixing characteristics. Its carrier-free formulation allows for an immediate start to the bone healing process.
ACTISTIM™ Demineralized Fiber Putty is made from 100% human allograft. The putty features an osteoinductive and osteoconductive 3D interwoven demineralized cortical fiber scaffold for improved handling, wicking, and mixing characteristics. Its carrier-free formulation allows for an immediate start to the bone healing process. -

 As a natural layer surrounding the fetus, the amniotic membrane is composed of two layers: the amnion – the layers closest to the fetus, and the chorion – the layers closest to the mother. Together, these layers provide a protective barrier to accommodate the growing fetus. The features of these layers make amnion chorion membranes ideal for homologous use as a barrier in a variety of applications.
As a natural layer surrounding the fetus, the amniotic membrane is composed of two layers: the amnion – the layers closest to the fetus, and the chorion – the layers closest to the mother. Together, these layers provide a protective barrier to accommodate the growing fetus. The features of these layers make amnion chorion membranes ideal for homologous use as a barrier in a variety of applications.