-
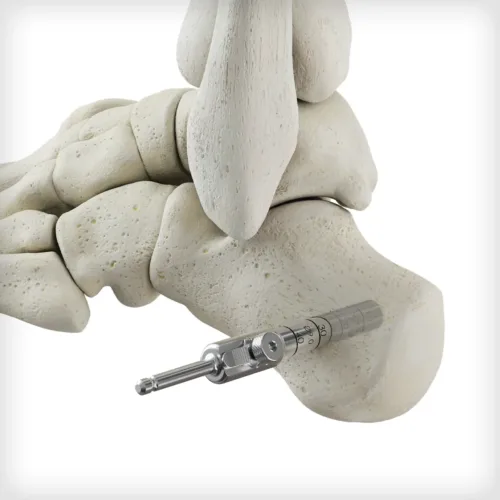
 ACTIGLASS™ Backfill Bioimplant combines the osteoconductive properties of tricalcium phosphate and hydroxyapatite with bioactive glass in a collagen matrix. Together, this biocompatible composition can resorb and be replaced by natural bone. This synthetic bioimplant is pre-sized for compatibility with the Autograft Harvester, allowing for improved intraoperative convenience and efficiency.
ACTIGLASS™ Backfill Bioimplant combines the osteoconductive properties of tricalcium phosphate and hydroxyapatite with bioactive glass in a collagen matrix. Together, this biocompatible composition can resorb and be replaced by natural bone. This synthetic bioimplant is pre-sized for compatibility with the Autograft Harvester, allowing for improved intraoperative convenience and efficiency. -
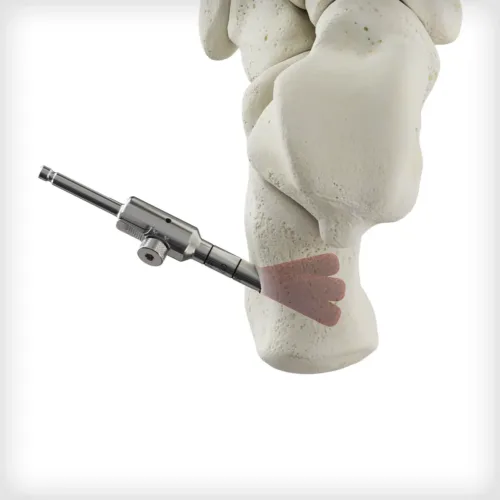
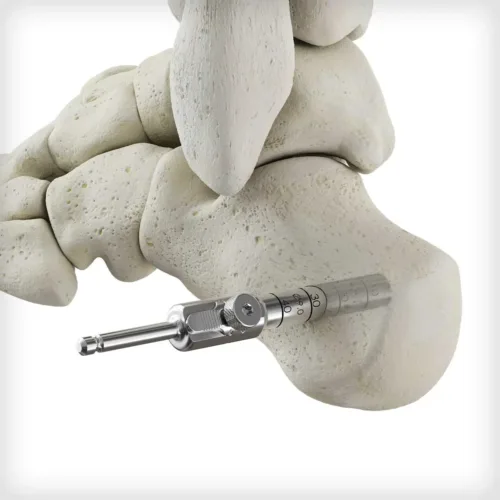
 The Autograft Harvester is a two piece instrument used to recover autogenous bone. The harvester connects to power via an AO quick connect and can be disassembled with a knob that accepts a T15 Driver. It contains a scoop feature to remove the graft from the housing. Multiple sizes are available based on patient anatomy or desired harvest site, including the calcaneus, proximal or distal tibia, and iliac crest. A synthetic bioimplant is available to backfill the void created by the harvester.
The Autograft Harvester is a two piece instrument used to recover autogenous bone. The harvester connects to power via an AO quick connect and can be disassembled with a knob that accepts a T15 Driver. It contains a scoop feature to remove the graft from the housing. Multiple sizes are available based on patient anatomy or desired harvest site, including the calcaneus, proximal or distal tibia, and iliac crest. A synthetic bioimplant is available to backfill the void created by the harvester. -
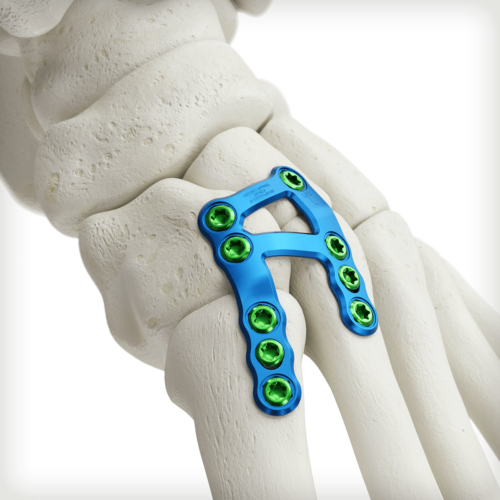
 1st/2nd TMT Lisfranc plates provide multiple points of fixation in the medial cuneiform and 1st and 2nd metatarsals along with a reinforcement strut from the medial cuneiform to the base of the second metatarsal. The tab-style variant features a machined groove on the medial cuneiform and distal 2nd metatarsal, which can be used as a bending zone or to cut the plate depending on patient anatomy. The tab-style plates include a longer, solid bridge section over the 2nd TMT joint to allow for placement of an independent homerun screw targeting the base of the 2nd metatarsal.
1st/2nd TMT Lisfranc plates provide multiple points of fixation in the medial cuneiform and 1st and 2nd metatarsals along with a reinforcement strut from the medial cuneiform to the base of the second metatarsal. The tab-style variant features a machined groove on the medial cuneiform and distal 2nd metatarsal, which can be used as a bending zone or to cut the plate depending on patient anatomy. The tab-style plates include a longer, solid bridge section over the 2nd TMT joint to allow for placement of an independent homerun screw targeting the base of the 2nd metatarsal.
2nd/3rd TMT Lisfranc plates provide multiple points of fixation in the lateral cuneiform and 2nd and 3rd metatarsals along with an intermetatarsal reinforcement strut for stabilization. The anatomical contouring over the 3rd TMT section, added points of fixation, and multiple size options ensure a proper fit for a broad range of patients along with the benefit of intercuneiform stability. -

 Lisfranc screws are available in Ø3.7mm and Ø4.1mm options and feature a special corticocancellous thread pitch optimized for the anatomy and indication. The screws are type II anodized for increased fatigue strength and feature an optimized head size to minimize soft tissue irritation medially. The ratcheting and locking Lisfranc clamp aids in anatomical reduction and functions as a targeting guide, allowing for wire placement, drilling, countersinking, measurement, and screw placement to be performed through a single instrument for increased surgical efficiency and accuracy.
Lisfranc screws are available in Ø3.7mm and Ø4.1mm options and feature a special corticocancellous thread pitch optimized for the anatomy and indication. The screws are type II anodized for increased fatigue strength and feature an optimized head size to minimize soft tissue irritation medially. The ratcheting and locking Lisfranc clamp aids in anatomical reduction and functions as a targeting guide, allowing for wire placement, drilling, countersinking, measurement, and screw placement to be performed through a single instrument for increased surgical efficiency and accuracy. -
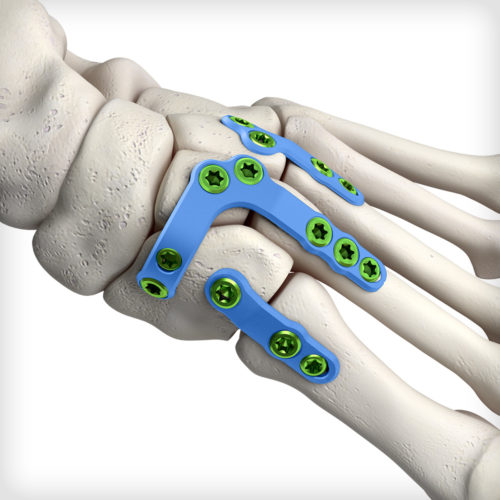
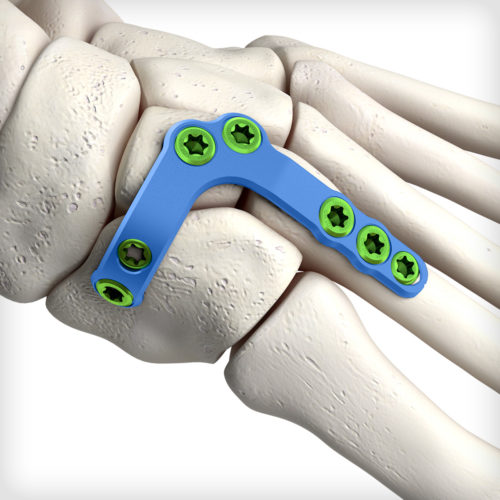 Unique deconstructed U-style Lisfranc plates address variations in injury pattern and patient anatomy. The non-constraining designs provide the intraoperative flexibility to choose the most appropriate construct for isolated TMT joints. The system comes equipped with an articulating pin distractor, a small joint arthrotome, and fluted joint fenestration drill pins to prepare the fusion site.
Unique deconstructed U-style Lisfranc plates address variations in injury pattern and patient anatomy. The non-constraining designs provide the intraoperative flexibility to choose the most appropriate construct for isolated TMT joints. The system comes equipped with an articulating pin distractor, a small joint arthrotome, and fluted joint fenestration drill pins to prepare the fusion site. -

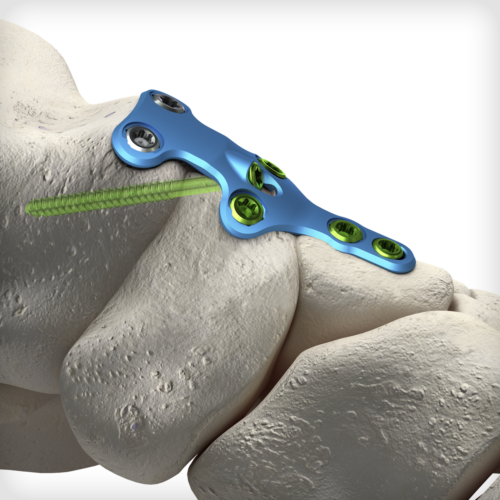 The uniquely contoured TNC (Talo-Naviculo-Cuneiform) Extension Revision plate is the first of its kind to address complex clinical scenarios involving the midfoot and hindfoot. The plate has been specially designed to fit dorsally and span the Navicular for TN non-union revisions, Navicular AVN (Mueller-Weiss Syndrome) cases, degenerative flatfoot cases with midfoot collapse/sag, and Lisfranc injuries that extend proximally through the NC/TN joints. The plate features our advanced dual-mode compression technology, giving the surgeon the freedom to select either traditional dynamic compression or cross-plate interfragmentary compression.
The uniquely contoured TNC (Talo-Naviculo-Cuneiform) Extension Revision plate is the first of its kind to address complex clinical scenarios involving the midfoot and hindfoot. The plate has been specially designed to fit dorsally and span the Navicular for TN non-union revisions, Navicular AVN (Mueller-Weiss Syndrome) cases, degenerative flatfoot cases with midfoot collapse/sag, and Lisfranc injuries that extend proximally through the NC/TN joints. The plate features our advanced dual-mode compression technology, giving the surgeon the freedom to select either traditional dynamic compression or cross-plate interfragmentary compression. -

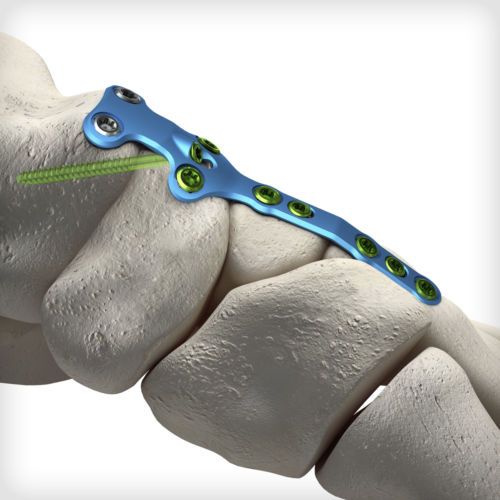 The uniquely contoured TNCM (Talo-Naviculo-Cuneiform-Metatarsal) Extension Revision plate is the first of its kind to address complex clinical scenarios involving the midfoot and hindfoot. The plate has been specially designed to fit dorsally and span the Navicular for TN non-union revisions, Navicular AVN (Mueller-Weiss Syndrome) cases, degenerative flatfoot cases with midfoot collapse/sag, and Lisfranc injuries that extend proximally through the NC/TN joints. The plate features our advanced dual-mode compression technology, giving the surgeon the freedom to select either traditional dynamic compression or cross-plate interfragmentary compression.
The uniquely contoured TNCM (Talo-Naviculo-Cuneiform-Metatarsal) Extension Revision plate is the first of its kind to address complex clinical scenarios involving the midfoot and hindfoot. The plate has been specially designed to fit dorsally and span the Navicular for TN non-union revisions, Navicular AVN (Mueller-Weiss Syndrome) cases, degenerative flatfoot cases with midfoot collapse/sag, and Lisfranc injuries that extend proximally through the NC/TN joints. The plate features our advanced dual-mode compression technology, giving the surgeon the freedom to select either traditional dynamic compression or cross-plate interfragmentary compression. -

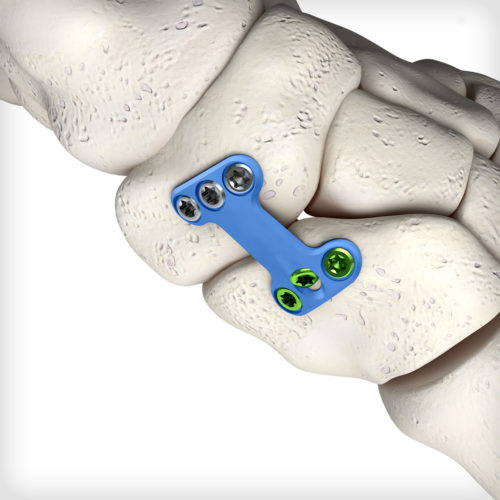 NC Fusion plates feature our advanced dual-mode compression technology, giving the surgeon the freedom to select either traditional dynamic compression or cross-plate interfragmentary compression. The system comes equipped with an articulating pin distractor, a small joint arthrotome, and fluted joint fenestration drill pins to prepare the fusion site.
NC Fusion plates feature our advanced dual-mode compression technology, giving the surgeon the freedom to select either traditional dynamic compression or cross-plate interfragmentary compression. The system comes equipped with an articulating pin distractor, a small joint arthrotome, and fluted joint fenestration drill pins to prepare the fusion site. -

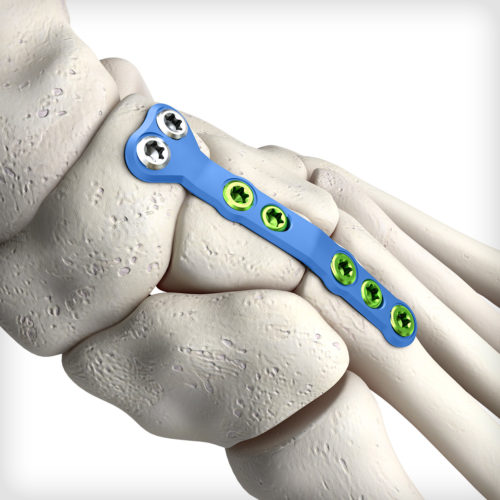 The uniquely contoured Middle Column Fusion plates are the first of their kind to address complex midfoot arthritis patterns with NC joint involvement. The system comes equipped with an articulating pin distractor, a small joint arthrotome, and fluted joint fenestration drill pins to prepare the fusion site.
The uniquely contoured Middle Column Fusion plates are the first of their kind to address complex midfoot arthritis patterns with NC joint involvement. The system comes equipped with an articulating pin distractor, a small joint arthrotome, and fluted joint fenestration drill pins to prepare the fusion site. -
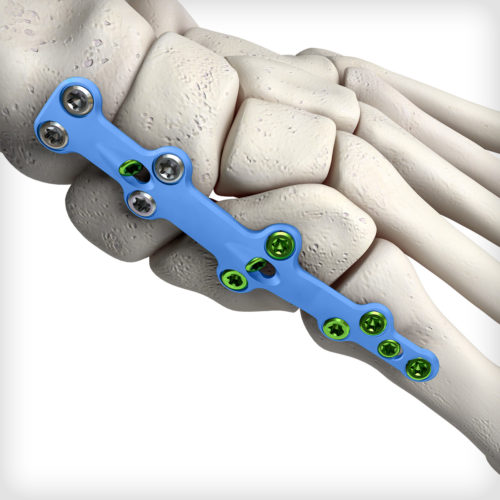
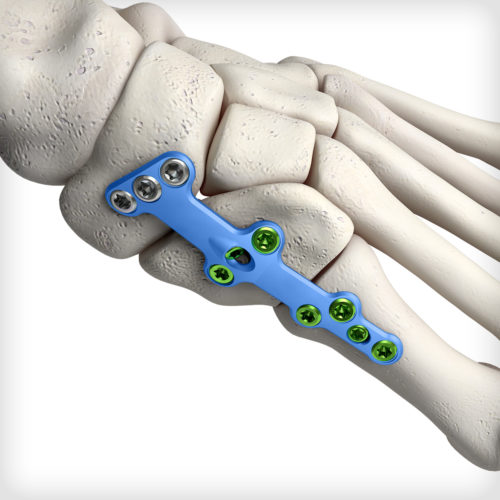 Medial Column Fusion plates feature our advanced dual-mode compression technology, giving the surgeon the freedom to select either traditional dynamic compression or cross-plate interfragmentary compression. Additionally, these plates are up to 2.5mm thick in certain sections and accommodate up to Ø4.0mm locking and non-locking screws for patients requiring more robust fixation. The system comes equipped with an articulating pin distractor, a small joint arthrotome, and fluted joint fenestration drill pins to prepare the fusion site.
Medial Column Fusion plates feature our advanced dual-mode compression technology, giving the surgeon the freedom to select either traditional dynamic compression or cross-plate interfragmentary compression. Additionally, these plates are up to 2.5mm thick in certain sections and accommodate up to Ø4.0mm locking and non-locking screws for patients requiring more robust fixation. The system comes equipped with an articulating pin distractor, a small joint arthrotome, and fluted joint fenestration drill pins to prepare the fusion site.


